configuration of nitrogen|Nitrogen : Baguio Wayne Breslyn. 794K subscribers. Subscribed. 556. 124K views 10 years ago. A step-by-step description of how to write the electron configuration for Nitrogen (N). In order to . To sign up to play or for help with your People’s Postcode Lottery account, call our Customer Experience team on 0808 109 8765 or email us at
[email protected]. Skip to Main Content £10 Prizes 29 August 2024. Search for £10 prizes announced on this date by entering your postcode. To check for .
configuration of nitrogen,The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
When we write the configuration we'll put all 19 electrons in orbitals around the .
Wayne Breslyn. 794K subscribers. Subscribed. 556. 124K views 10 years ago. A step-by-step description of how to write the electron configuration for Nitrogen (N). In order to .
The electron configuration of nitrogen refers to the arrangement of electrons in the nitrogen atom’s orbitals. It describes how electrons are distributed among the various atomic .
Nitrogen is cycled naturally by living organisms through the ‘nitrogen cycle’. It is taken up by green plants and algae as nitrates, and used to build up the bases needed to construct DNA, .
The electron configuration of nitrogen is 1s22s22p6. Nitrogen becomes present in most proteins, playing a very important role in certain biochemical applications, such as industrial applications.Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at . A single Nitrogen atom has 7 protons and 7 electrons, but how do we know where Nitrogen puts its electrons.
The full electron configuration for nitrogen is "1s"^ 2"2s"^2"2p"^3. The noble gas shorthand electron configuration is ["He"]"2s"^2"2p"^3". The atomic number of nitrogen is 7. .
The electron configuration of nitrogen is 1s2 2s2 2p3. You can see the image below. What is the Orbital Diagram for Nitrogen? For drawing the orbital diagram configuration of the nitrogen we have to write the following: .

Electron Configuration. [He] 2s 2 2p 3. Nitrogen is present in all living organisms, in proteins, nucleic acids and other molecules. Physical Properties. Phase. Gas.
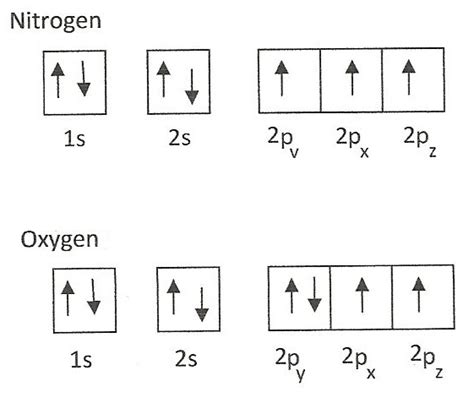
configuration of nitrogen Nitrogen The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration.
The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration.Isotopes. Nitrogen has two naturally occurring isotopes, nitrogen-14 and nitrogen-15, which can be separated with chemical exchanges or thermal diffusion. Nitrogen also has isotopes with masses of 12, 13, 16, and 17, but they are radioactive.. Nitrogen 14 is the most abundant form of nitrogen and makes up more than 99% of all nitrogen found on Earth.It is a stable compound .Nitrogen Nitrogen Electron Configuration: N is one of the chemical element that has a symbol N. The atomic number of nitrogen is 7. A Scottish physician Danial Rutherford discovered and isolated nitrogen in the year 1772. However, Henry Cavendish and Carl Wilhelm Scheele had independently during the same time but Rutherford is generally accorded the . Let's find the electron configuration of Nitrogen! A single Nitrogen atom has 7 protons and 7 electrons, but how do we know where Nitrogen puts its electrons.
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Nitrogen (N) [He] 2s 2 2p 3: 1s 2 2s 2 2p 3: 2, 5: 8: Electron configuration of Oxygen (O) [He] 2s 2 2p 4: 1s 2 2s 2 2p 4: 2, 6: 9: Electron configuration of . The electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have unpaired spins. The nitrogen family includes the following compounds: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). All Group 15 elements have the electron configuration ns2np3 in . Group 15: General Properties and Reactions - . Let us return to the electron configuration of nitrogen and write it again: Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Otherwise, write the order of the energy levels with electron configuration chart: 1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3. The full electron configuration for nitrogen is "1s"^ 2"2s"^2"2p"^3. The noble gas shorthand electron configuration is ["He"]"2s"^2"2p"^3". The atomic number of nitrogen is 7. This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So the electron configuration will include 7 electrons placed . Consider the correct electron configuration of the nitrogen (Z = 7) atom: 1s 2 2s 2 2p 3. The p orbitals are half-filled; there are three electrons and three p orbitals. This is because the three electrons in the 2p subshell will fill all the empty orbitals first .
configuration of nitrogenBonding in NH 3. The nitrogen in NH 3 has five valence electrons. After hybridization these five electrons are placed in the four equivalent sp 3 hybrid orbitals. The electron configuration of nitrogen now has one sp 3 hybrid orbital completely filled with two electrons and three sp 3 hybrid orbitals with one unpaired electron each. The two electrons in the filled sp 3 hybrid orbital are .Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating .
The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.The chemical symbol for Nitrogen is N. Electron Configuration and Oxidation States of Nitrogen. Electron configuration of Nitrogen is [He] 2s2 2p3. Possible oxidation states are +1,2,3,4,5/-1,2,3. Electron ConfigurationThe atomic number of nitrogen is 7, which means it has 7 electrons. Now it is possible to find the orbital notation of nitrogen very easily through electron configuration. That is, the orbital notation of nitrogen is 1s 2 2s 2 2p 3. What is Hund’s principle?

The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the .In this study, we investigate the oxygen reduction reaction (ORR) reactivity of nitrogen-doped graphene by using density functional theory (DFT), a computational quantum mechanical technique. Four doping configurations and five models were comprehensively studied: quaternary nitrogen (NQ), pyrrolic nitrogen Editors’ collection: Graphene
configuration of nitrogen|Nitrogen
PH0 · What is the electron configuration of nitrogen?
PH1 · Nitrogen Electron Configuration (N) with Orbital Diagram
PH2 · Nitrogen Electron Configuration
PH3 · Nitrogen
PH4 · How to Write the Electron Configuration for Nitrogen and N3
PH5 · Electron configuration of nitrogen
PH6 · Electron Configuration for Nitrogen (N)